Die einfache Erklärung: Kalk lernt schwimmen.
Kalk im Wasser besteht hauptsächlich aus den Mineralien Calcium und Magnesium. Anstatt diese wertvollen Mineralien Calcium und Magnesium (Kalk) dem Wasser zu entziehen, reduziert AQON PURE das Anlagerungsverhalten von Kalk im Wasser. Kalk liegt im Trinkwasser in gelöster Form als Calcium- (Ca2+) und Carbonat-Ionen (CO32-) vor. Strömt das Wasser durch AQON PURE, berühren diese Ionen den im AQON PURE verbauten Katalysator. Die Calcium- und Carbonat-Ionen reagieren auf der katalytischen Oberfläche und bilden kleine Kalkkristalle (Calciumcarbonat CaCO3).
Die gebildeten Kalkkristalle dienen als Anlagerungspunkt für die übrigen Calcium- und Carbonat-Ionen im Wasser. Anstatt also Kalkablagerungen auf Oberflächen wie z.B. Rohrleitungen zu bilden, reagiert der Kalk mit sich selbst. Der Vorteil: Der Mineralgehalt des Wassers (die Wasserhärte) wird dabei nicht reduziert. Die gesamte Reaktion läuft ohne die Zugabe von chemischen Stoffen ab, da sich der Katalysator nicht verbraucht. Vereinfacht gesagt: Ihr Kalk lernt schwimmen, lagert sich dadurch weniger ab, und wird bei jeder Wasserentnahme einfach ausgeschwemmt. Dadurch werden Kalkablagerungen wirksam reduziert.
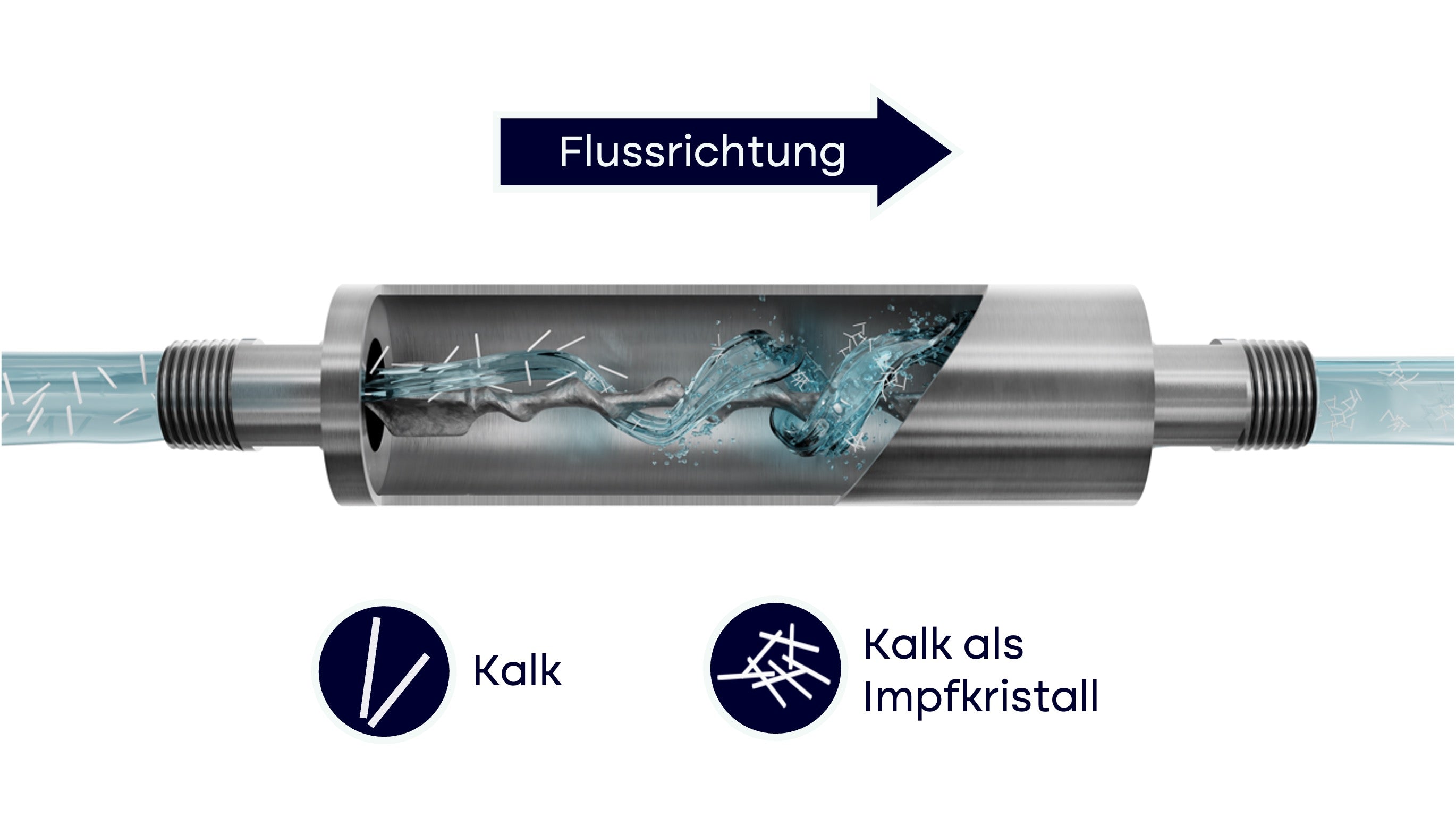
Funktionsmodell: AQON PURE funktioniert nach dem Prinzip der Impfkristallbildung
AQON PURE ist eine Enthärtungsanlage ohne Salz, die auf dem Prinzip der Impfkristallbildung funktioniert. Statt wertvolle Mineralien zu entziehen, reduziert sich das Anlagerungsverhalten von Kalk im Wasser. Die Folge: Weniger Kalkablagerungen auf Oberflächen und keine Belastung der Umwelt durch die Abgabe von Chlorid.
Was ist Kalk?
Kalk ist ein natürliches Mineral, das hauptsächlich aus Calciumcarbonat (CaCO₃) besteht. Es kommt in verschiedenen Formen vor, wie zum Beispiel in Kreide, Marmor oder Quellkalk. Ganz anders sieht die Kombination aus Kalk und Haushaltsgeräten aus. Erhitztes kalkhaltiges Wasser hinterlässt nicht nur unschöne Kalkflecken auf Oberflächen, kalkhaltiges Wasser ist oftmals der Grund, wieso Elektrogeräte nicht mehr funktionieren und ersetzt werden müssen.
Ist Kalk gesundheitsgefährend?
Kalk an sich ist in der Regel nicht gesundheitsgefährdend, solange er in angemessenen Mengen und unter normalen Bedingungen verwendet wird. Calciumcarbonat, das Hauptbestandteil von Kalk, wird oft als Nahrungsergänzungsmittel eingesetzt, um Calcium zu liefern, und ist für die meisten Menschen unbedenklich.
Was ist die Wasserhärte?
Wasserhärte beschreibt, wie viele Mineralien, hauptsächlich Calcium und Magnesium, im Wasser gelöst sind. Hartes Wasser enthält viele dieser Mineralien, was Auswirkungen auf Haushaltsgeräte und den Seifenverbrauch haben kann.
Reduziert AQON PURE die Wasserhärte?
Nein, AQON PURE reduziert nicht die Wasserhärte. Durch die Impfkristallbildung wird lediglich das Anlagerungsverhalten von Kalk verändert. Der Vorteil: Wertvolle Mineralien bleiben im Trinkwasser enthalten. Gleichzeitig sorgt das veränderte Anlagerungsverhalten dazu, dass weniger Kalkablagerungen auf Oberflächen entstehen.
Die wissenschaftliche Erklärung: Impfkristallbildung
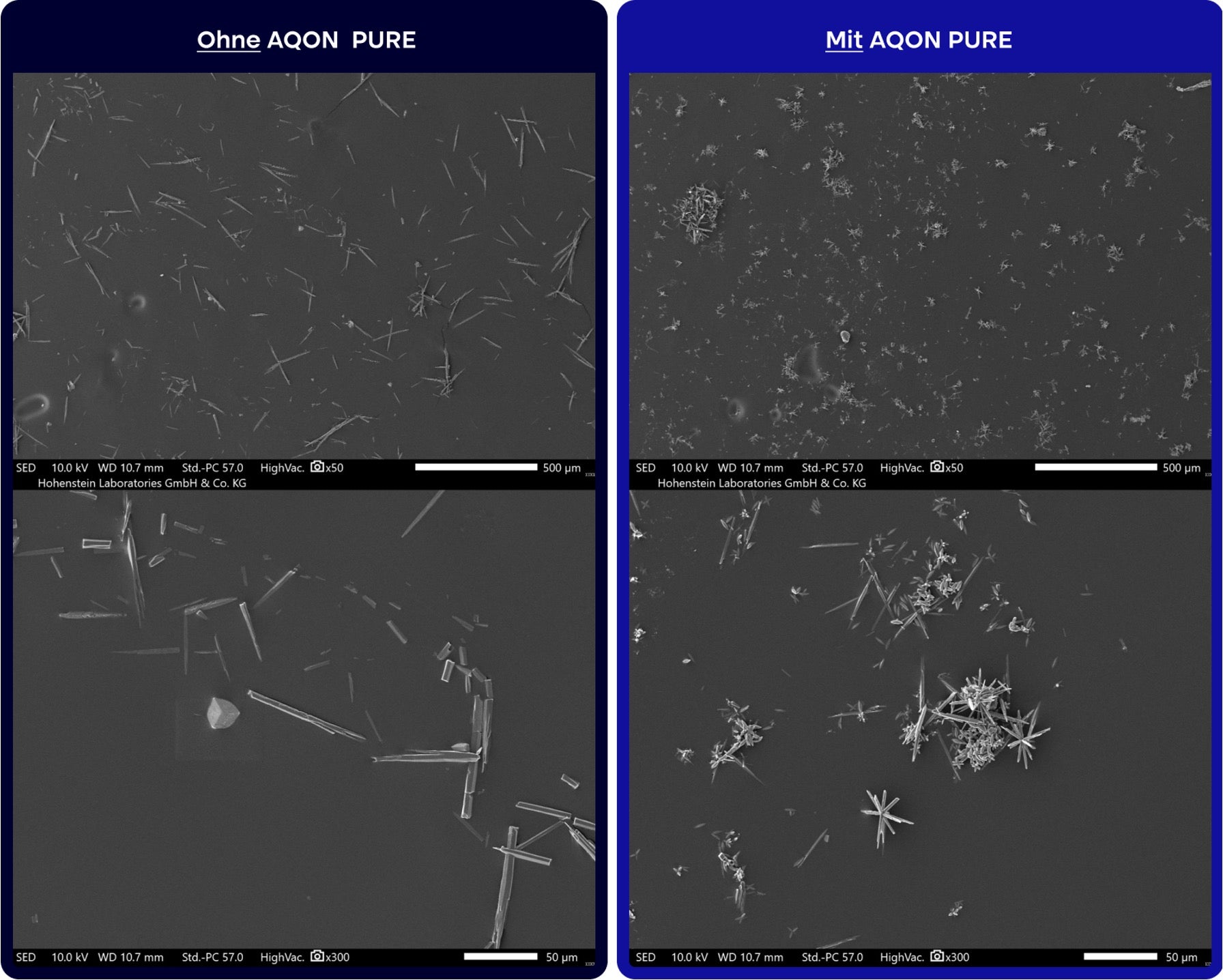
Mikroskopische Aufnahmen: Abbildung aus Bericht Nr. 22.8.6.0003 vom 25.05.2022: Aufnahmen durch die Hohenstein Innovations gGmbH zum Vergleich der Kalk-Kristalle mit und ohne Behandlung durch das AQON PURE Modul. Die EDX-Spektren der Kristallstrukturen zeigen auf, dass im durch AQON PURE behandelten Wasser größere Kalkinseln in Form von Aragonit Kalkkristallen gebildet wurden (Impfkristalle). Bitte beachten Sie, dass es sich bei der Darstellung der durch AQON PURE gebildeten Impfkristalle um eine 50-fache und 300-fache Vergrößerung handelt. Die gebildeten Impfkristalle sind für das menschliche Auge nicht zu erkennen und können auch kein “Verstopfen“ der Leitungen o.ä. zur Folge haben.
Neben der katalytischen Impfkristallbildung gibt es noch weitere Verfahren zur Impfkristallbildung, die ihre Wirksamkeit in der Praxis bewiesen haben [1]. Die Effektivität der chemiefreien Wasserbehandlung ist bereits auch durch renommierte Wissenschaftler belegt [2, 3, 4] (u. a. durch katalytische Wirkung oder durch Einwirkung von elektromagnetischen Feldern [5]) und folgt folgenden grundlegenden Prinzipien:
Wie oben bereits beschrieben, liegt Kalk im Trinkwasser in gelöster Form als Calcium- (Ca2+) und Carbonat-Ionen (CO32-) vor. Durch die katalytische Wirkung von bestimmten Metallen [2, 4] wird das Wachstum der Calcium-Kristallkeime beeinflusst und ein frühes Ausfallen der Kristallkeime in Aragonit-Form begünstigt. Dieser Prozess erfolgt im Fall der AQON PURE-Technologie durch katalytische Effekte, bei denen spezielle Metalle eine Adsorption und eine sogenannte Co-Fällung von Kristallkeimen initiieren. [2]
Diese Katalysator-Metalle werden jedoch nicht verbraucht (nicht an das Trinkwasser abgegeben), sondern beschleunigen Reaktionen, die zur Calcium-Keimbildung führen. Bei diesem Prozess wird das Kalk-Kohlensäure-Gleichgewicht gestört, indem Bestandteile wie Carbonat-Ionen mit den Metallgrenzflächen wechselwirken. Verschiebt sich das chemische Gleichgewicht (z. B. Überschuss an Hydrogencarbonat-Ionen), so wird auch der pH-Wert beeinflusst, welcher sich z. B. „minimal erhöht“. [2, 6] Der Begriff „Calciumhydrogencarbonat-Kristalle“ spiegelt die Erkenntnisse zur Steuerung der Keimbildung von Calcium-Kristallen wider (wir verstehen hierunter nur metastabile Zustände im Keimbildungsprozess, was jedoch schwierig für ein allgemeines Publikum darstellbar ist). Hierbei ist das Ziel, Begrifflichkeiten zu wählen, die die besondere Rolle von Hydrogencarbonat-Ionen innerhalb des Keimbildungsprozesses von Kalkkristallen (u. a. Einfluss auf Struktur der Kalkkristalle) umschreiben und betonen. [6]
Die im AQON PURE System verwendete Technologie wurde in einer 18-monatigen Studie auf seine Wirksamkeit untersucht [7]. Außerdem führte Hohenstein Wirksamkeitsuntersuchungen un Bezug auf die Impfkristallbildung durch. Beide Untersuchungen können in unserem Bereich "Wirksamkeit" eingesehen werden.


Der Unterscheid zur herkömmlichen Wasserenthärtung mit Salz.
Der größte Unterschied der Impfkristallbildung zur herkömmlichen Wasserenthärtung mit Salz besteht darin, dass die Impfkristallbildung kein Salz bzw. Natrium an das Trinkwasser abgibt. Eine klassische Wasserenthärtungsanlage mit Salz (Natriumchlorid), entzieht dem Wasser diese Mineralien und tauscht sie gegen Natrium aus. Dadurch sinkt der Mineralgehalt (Kalkgehalt) des Leitungswassers und der Natriumgehalt steigt. Eigentlich ein Widerspruch, oder? Im Supermarkt achten wir schließlich auch darauf, dass wir Wasser kaufen, das reich an den natürlichen Mineralien Calcium und Magnesium (Kalk) ist, und natriumarm. Wer also bisher auf eine klassische Enthärtungsanlage mit Salz setzte, um Kalkablagerungen zu reduzieren, verschlechterte seine Trinkwasserqualität. Gleichzeitig geben Wasserenthärtungsanlagen mit Salz große Mengen Chlorid durch ihr Abwasser ab, was wiederum schlecht für die Umwelt ist. Darüber hinaus sind Wasserenthärtungsanlagen sowohl in der Anschaffung als auch im Betrieb teurer als Enthärtungsanlagen ohne Salz. Außerdem kann das Ionenaustauscherharz eines Wasserenthärters mit Salz, insbesondere bei längeren Stillstandszeiten, das Risiko einer Verkeimung des Trinkwassers erhöhen. Aus diesem Grund müssen Enthärtungsanlagen mit Salz jährlich durch Fachpersonal gewartet und gereinigt werden. Außerdem darf man nicht vergessen, dass bei Wasserenthärtungsanlagen mit Salz regelmäßig Regeneriersalz nachgefüllt werden muss (für ein Einfamilienhaus mit 4 Personen ca. 100 kg pro Jahr).
Was funktioniert besser? Wasserenthärtung mit Salz oder Enthärtung ohne Salz?
Eine abschließende Bewertung dieser Frage ist nur schwer möglich. Auch fehlt es bisher an Vergleichstests beider Verfahrensweisen. Denn auch wenn eine Wasserenthärtungsanlage mit Salz den Kalkgehalt (die Wasserhärte) des Leitungswassers reduziert, so liefern diese Anlagen kein kalkfreies Wasser. Aus Gründen der Korrosion belässt man bei einer Wasserenthärtungsanlage mit Salz immer eine sogenannte Restwasserhärte (Restmenge an Kalk) im Wasser.
Sowohl diese Restwasserhärte einer Enthärtungsanlage mit Salz, als auch der veränderte Kalk durch das Verfahren der Impfkristallbildung führen nach einer gewissen Zeit zu Kalkspuren auf Oberflächen, wenn Wasser z. B. verdunstet. Auch müssen Küchengeräte wie z. B. der Wasserkocher zwar seltener, aber dennoch nach einer gewissen Zeit entkalkt werden. Vermutlich bei der Verwendung einer Wasserenthärtungsanlage mit Salz noch etwas seltener. Demgegenüber stehen jedoch die Vorteile einer Enthärtungsanlage nach dem Prinzip der Impfkristallbildung in Bezug auf Anschaffungskosten, Betriebsaufwand, Trinkwasserqualität und Umweltfreundlichkeit.
Zusammengefasst: Beide Verfahren reduzieren Kalkablagerungen im Haus und machen das Leben mit Kalk in den eigenen vier Wänden angenehmer. Das Putzen bleibt jedoch in beiden Fällen nicht erspart, fällt jedoch leichter.
[1] B. Wricke, W. Baumgardt, „Stand der Technik auf dem Markt verfügbarer alternativer Anlagen zur Vermeidung bzw. Verminderung der Steinbildung im Warmwasserbereich“, DVGW-Publikationen, 2003.
[2] Ruelo et al., „Assesing the effect of catalytic materials on the scaling
of carbon steel“, Desalination, 313, 189–198, 2014.
[3] Tijing et al. „Effect of high-frequency electric fields on calcium
carbonate scaling“, Desalination, 279, 47–53, 2011.
[4] Coetzee et al., Scale reduction and scale modification effects
induced by Zn and other metal species in physical water treatment“, Water SA, 24,77–84, 1998.
[5] Lin et al., „A critical review of the application of electromagnetic fields for scaling control in water systems: mechanisms, characterization, and operation“, npj Clean Water, 35, 1–9, 2020.
[6] Huang et al., „Uncovering the Role of Bicarbonate in Calcium
Carbonate Formationat Near-Neutral pH“, Angewandte Chemie. Int. Ed., 60, 16707–16713, 2021.
[7] Howett, D. (2015). AWT: Catalyst-Based Scale Prevention for Domestic Hot Water Systems (Overviews): https://www.gsa.gov/climate-action-and-sustainability/center-for-emerging-building-technologies/completed-assessments/water/awt-catalystbased-scale-prevention






